About the mechanism of antibody production, says S.Ananthanarayanan.
The efficacy of vaccines against disease and how fast they create antibodies have become topics of interest. We have the processes to create the vaccines in the lab, but how the body uses the vaccine to create immunity, and how the process is controlled, is not understood in detail.
Saumya Kumar, Válter R. Fonseca, Filipa Ribeiro, Afonso P. Basto, Ana Água-Doce, Marta Monteiro, Dikélélé Elessa, Ricardo J. Miragaia, Tomás Gomes, Eliane Piaggio, Elodie Segura, Margarida Gama-Carvalho, Sarah A.Teichmann, and Luis Graca, from the University of Lisbon, Hospital de Santa Maria, Lisbon, Instituto Gulbenkian de Ciência, Oeiras, Portugal, University of Cambridge and Wellcome Sanger Institute, Cambridge and Institut Curie, Paris, describe in the journal, Science Immunology, their work, with the processes within the cells that create antibodies, and the factors that control the processes. These are the processes that vaccines set in motion, and also the reasons for autoimmune diseases, like forms of diabetes, multiple sclerosis and rheumatoid arthritis. Understanding the mechanism of the process could be a game changer.

The immune reaction of the body starts with the action of special cells which engulf foreign particles and extract and display characteristic portions of the invader, to enable other immune-reaction cells recognize and respond to invaders. These special cells are called antigen presenting cells, and the cells that receive their information are of two kinds, the B cells and the T cells. While T cells either act to destroy body cells that are infected by the antigen, or to communicate with B cells, the B cells produce antibodies, which are special proteins that can neutralize the antigen itself.
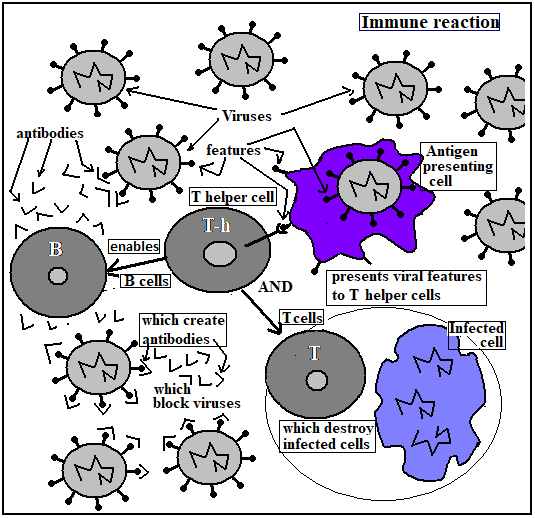
The B cells and T cells originate from stem cells, in the bone marrow. After development in the bone marrow, the cells migrate, through the blood, to the spleen or the thymus, for maturation and activation. In mammals, the B cells migrate to the spleen. B cells, historically, were first discovered in birds, and in birds, the B cells migrate to an organ called the bursa. This is the reason that the cells are called ‘B’ cells. T cells get their name from the thymus, where they migrate for maturation.
The region of the organs, such as the spleen, in the case of B cells, where the cells complete maturation, is called the Germinal Centre. These organs receive a constant stream of antigens, which bind to B cells, in the case of the spleen, by means of a surface structure known as the B cell receptor and the binding takes place with the help of cells called T helper cells. The B cells then undergo rounds of cell division and produce antibody proteins, which, by a process of selection, are sculpted into the correct shape to fit the key surface features of antigens, such as viruses. It is thanks to these features that viruses are able to gain access to the cells that they infect. If the antibody molecule attaches to the virus and blocks this vital feature, the virus can no longer enter its target cell, which is to say, it cannot cause disease.
In addition to the T helper cells, which promote B cell processes that lead to antibodies, the Germinal Centre has T regulator cells, whose role appears to be restriction of T helper cells and the division of B cells. Their purpose would hence be to enable selective, greater production of the most suitable antibody variation. The fact of the matter is that even when not stimulated by antigens, many trillions of antibodies are possible and do exist. As one antibody can deal with more than just one pathogen, this collection of antibodies is an arsenal that could handle, and with some accuracy, a great many pathogens. When a pathogen does appear, however, and repeatedly, as in infection, B cells refine their antibodies for higher affinity, to deal with the antigen much more effectively, a process called affinity maturation. And this process is orchestrated by changes of the balance in the activity of T helper cells and T regulator cells.
The paper in Science Immunology says studying this balance of the two kinds of T cells has not been possible so far, because there is no way to simulate in the laboratory the process that takes place within living cells. This is apart from difficulties in accessing cells of organs like the spleen and associated systems. The authors of the paper employed a collection of techniques, which are most recent developments that have only now become available, of measuring gene expression in bulk and then making estimates of the genetic expression of individual cells. Estimation of genetic expression is important because the process of generation of antibody proteins by B cells involves the shuffling of genes, or the portions of the DNA of the cells, which code for proteins. And it is the effect of T helper and T regulator cells in this process, that promote or retard the production of particular antibody proteins.
Genes lead to formation of proteins through a process of copying themselves to molecules called RNA, which then convey the genetic information to agencies that assemble proteins. The methods of identifying RNA are now in the news, as it is an RNA that the COVID-19 virus contains. The RNA, and hence the genes related to antibodies are also identified in the same way. However, there is a difference - in detecting COVID-19, only a known RNA needs to be detected. But for studying the growth of antibodies there are thousands of RNA concentrations arising from different cells and the task is to discover how the mix of T cells affects their growth and the promotion of desired antibodies.
The technique used, known as single-cell transcriptomics, is truly state-of-the-art, a collection of methods to mark, label, sort, identify gene expression in thousands of cells, and simultaneously. The data collected is analysed using sophisticated computational techniques, and a seemingly impossible task of arriving at single-cell expression profiles is achieved. And, using these methods, the group was able to work out a scheme of how T helper and T regulator cells arise, and the mechanics of how antibody production is regulated.
The pace of antibody production resulting from different COVID-19 vaccines have been in the news now for several weeks. Some vaccines are found to be more effective than others, and modifying the time-gap between the first and second dose of the vaccine has been found to affect the level of antibody production. The news does not suggest that there is anything more than statistical or anecdotal data to rely on. The current study would bring about a change, and enable more directed paths of enquiry into how vaccines could be more effective. Understanding the internals of the immune reaction would also lead to methods to control excessive or unwanted generation of antibodies, as happens in auto immune diseases.
------------------------------------------------------------------------------------------ Do respond to : response@simplescience.in-------------------------------------------