
|
And with the discovery of the atom, the role of things atomic in the pressure and temperature of a gas, the way atoms emitted light and improvements in the quality and power of telescopes, by the beginning of the 20th century, the subject of astrophysics could be said to have come into being. It was in this exciting period that Meghnad Saha, of Kolkata University, did important work that helped put astrophysics on sound theoretical foundations, |
|---|
By the beginning of the 20th century, the stars, the sun included, were seen to have formed by the action of gravity between the billions of atoms in a vast cloud of gas, mostly hydrogen. As the gas got compressed, under the huge forces drawing everything towards the center, the gas heated up to millions of degrees. Under the intense heat, the atoms of hydrogen were squeezed close enough to fuse into helium, with the release of immense energy. The intense nuclear fire impelled expansion again, till the gas cooled, was compressed by gravity again, and so on.
Over the ages, this process resulted in the creation of all the heavier elements too, practically all of which have been detected in the sun and the stars. An identifying feature of elements is the exact colour of the light that atoms of the element emit, when they are excited. The presence of an element in a star can hence be detected by examining the light that comes from the star and looking for the spectral signature of the element, something like its fingerprints!
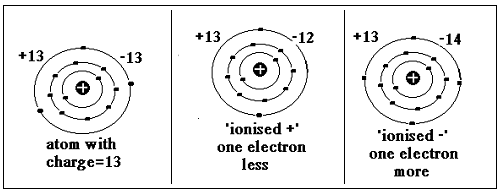
Atoms of the elements consist of a heavy core, with positively charged protons (and neutral neutrons), surrounded by a cloud of an equal number of negatively charged electrons. The electrons are very small and could be thought of as orbiting the core, rather like the planets orbit the sun. It is the number of protons and neutrons that account for the mass of the atom, while the charge in the nucleus, which is the number of protons and also electrons, decides the chemical properties of the atom.
There is a rule of the distribution of the electrons, in ‘shells’ around the nucleus. The first rule is that the outermost shell can have no more than 8 electrons. If the element has one more electron, then the electron starts a new shell with one electron, and so on. Atoms also strive to have 2 or 8 electrons in the outer shell. How can they do this? Well, in chemical combination, an element with 3 outer shell electrons may ‘lend’ on electron to another element with 7 in the outer shell, so that they both attain 2 and 8 in the outer shell. But as the number of electrons is now different from the charge on the nucleus, the atom would be charged, or in a state of ionization.
Electrons in shells all have energies, which keep them in their shells, and away from the nucleus, that attracts them. As electrons in the outer shells have been drawn further away from the nucleus, these electrons have more energy than electrons in the inner shells. Electrons can also be temporarily promoted to higher shells, by being given some energy through some form of kick, like the impact of a particle of light, with the energy that separates the two shells. This higher energy state is a temporary one and the atoms would spontaneously de-excite, by emitting a photon, or particle of light, of exactly the energy difference between the two states, in a very short time.
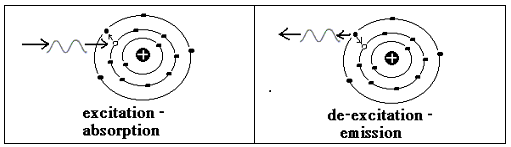
It is this energy of de-excitation, characteristic of the energy states of each atom, which decides the particular wavelength, or colour of the light that the atom emits. These colours of the everyday elements are easily in evidence. For instance, common salt is sodium chloride. Sodium has a characteristic yellow emission, which we can see just by sprinkling some common salt on to the kitchen gas flame. If we sprinkled some chalk, instead, we would see a red flame. This is because chalk is calcium carbonate and red is the colour characteristic of calcium. The heat of the flame only creates the easiest excitation of the atoms, which is of the outermost electrons, and shows only the colours of basic transitions. Many more colours that are characteristic get emitted with more vigorous excitation, at very high temperature, like during an electric arc or in sparking, when electrons in inner orbits participate.
The light emitted by all elements, under excitation, has been analysed by using sensitive prisms, which splits the light up into its constituents. In the case of sunlight, which has all the colours, the spectrum is a series of continuous bands of light. But in the emission from atoms, only specific colours are present and the spectrum is a set of lines, each line representing the characteristic wavelength of the light wave.
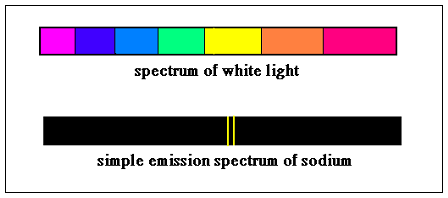
Just as atoms can emit at these characteristic colours de-exciting, they also absorb light at these same colours to get excited. Thus, if white light, which has all the colours, is shone through a gas of a substance that has particular emission lines, then, we could expect that these very colours would get absorbed by the gas, to be emitted again, in all directions. Thus, if we analysed the spectrum of the light emerging from the gas, we should find gaps where the emissions lines would appear, and that is exactly what we do see! The characteristic absorption bands of all elements have been carefully mapped and now a picture of the spectrum is a sensitive and reliable indicator of what elements are present in the medium through which any light has come.
The emission and absorption spectra from stars thus became important investigation tools, which revealed details of the structure of the sun, which helped refine our understanding.
Apart from this emission of light at particular wavelengths due to de-excitation of electrons, hot objects also emit a continuous spectrum, because of the vibration of their constituent parts. This is the nature of the composite, white light that the sun radiates, from the photosphere, the visible part, at some 6000°C. But when the spectrum of this light is studied in detail, it is seen that the spectrum is crossed by black lines! These are clearly because of the elements in the outer atmosphere of the sun having absorbed the particular colours. This kind of analysis has shown that the sun contains all the elements that are known on the earth and also that almost all stars are also made of the same elements as our sun!
Coming back to the structure of the sun, it was considered, as we had said, to be a gas, compressing itself under its own gravitational pressure, which was compensated by the impulse to expand, because of the heat generated at the core. The behaviour was thus like our own atmosphere, with the pressure due to the atmosphere being greater as we near the core and less at the periphery. The temperature, also, would be greater nearer the core and less at the periphery.
The structure, in fact, is now accepted as consisting of a very hot core, fueled by nuclear reactions, surrounded by an intermediate region of turbulence and convection currents. Outside this region is the photosphere, the region that we can see. Beyond the photosphere is the chromosphere, a region of thin gas, and yet beyond that is the corona. These outer regions are normally not visible, because of the dazzle of the photosphere and we need to wait for a total solar eclipse to get a glimpse.
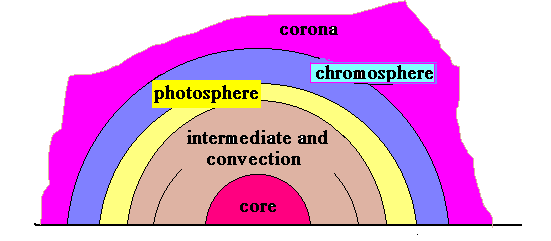
During an eclipse, the moon’s disc covers the bright photosphere and allows the comparatively less intense outer regions to be viewed or photographed. In the normal case, what we see is the absorption spectrum of the chromosphere. But in an eclipse, with the photosphere cut off, we are able to see emission wavelengths of the chromosphere and this is most revealing!
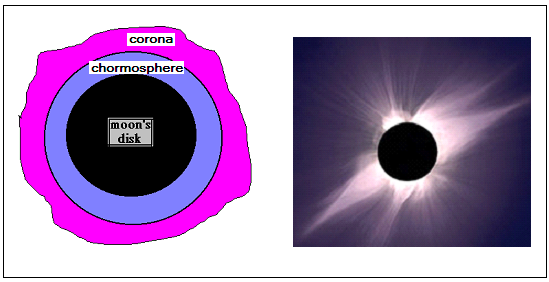

|
During what is called ‘flash photography’ of the chromosphere and corona, in the short minutes that they are visible, a remarkable discovery was the higher energy emission spectrum of calcium, an element 40 times as heavy as hydrogen, but no emission spectrum of hydrogen itself. And then, there were many other elements whose absorption spectra were missing in the chromosphere! It is possible to map the spectrum of different parts of the chromosphere and it was seen that hydrogen seemed to be present till a height of about 8000 km, but calcium was there at 14,000 km. And furthermore, the lines of calcium seen at this high altitude were the higher energy emissions, which were expected only at higher temperatures! |
|---|
The world’s astrophysics community was in confusion. How could the heavier calcium be present at higher altitudes than hydrogen? And how could the temperature seem to increase as one moved away from the central source of the heating? Like scientists the world over, Meghnad Saha, then at Kolkata University, was also seized of the problem. But thanks to his own background in the theory of chemical reactions and the subject of thermodynamics, he now moved in with an important and new idea.
Meghnad Saha was born in 1893 in a small village not far from Dacca, then part of the province of Bengal in British India. The family was of very ordinary means and it is thanks to a series of fortunate interventions by relatives and well wishers that young Meghnad was able to go even beyond primary school. But he was consistently a brilliant student in the village school, the English medium school in a nearby village and then at Dacca, with a free-ship and a stipend, where he prepared for entrance to college. He lost these benefits for taking part in a demonstration against the British Governor, at the age of 14. Fortunately, he was able to continue his studies, which included the German language, with other financial aid, till he joined Presidency College in Kolkata, at the age of 18.
At Kolkata, Meghnad was a classmate of S.N.Bose and P.C.Mahalnobi, who became celebrated scientists, and one of his teachers was J.C.Bose himself. Meghnad had a scintillating college career, but at its end, like so many Indians of the time, he tried to get into the civil service, so that he could help his family. Fortunately for physics, Meghnad was not considered to have impeccable political credentials and he ended up in Kolkata University, with S.N.Bose, thanks to Sir Ashutosh Mukherjee.
In the university physics department, S.N.Bose and Saha strove to introduce the latest topics into the curriculum. The two were ultimately given a special allowance and assigned subjects to read up and master, Saha being assigned Quantum theory. Thanks to his knowledge of German and with the help of books and journals, Bose and Saha were soon up to date with the latest in the world of physics and started actively pursuing research at Kolkata University. In 1920, which is soon after he joined the university and at the age of 27, Saha published his celebrated paper on the anomalous radiation seen in the flash photographs of the chromosphere!
To recapitulate, the puzzle in the study of the sun was, how did the upper chromosphere show the highest energy parts of the calcium emission spectrum, although lower altitudes, where the temperature should have been higher, did not? Did this imply that the sun’s atmosphere got hotter as one went higher? And why, in this higher part of the chromosphere, were many other elements absent? And finally, how was it that calcium, 40 times heavier than hydrogen, was present at much greater heights?
Explanations had been attempted, and workarounds had been tried, but the problem remained, particularly the one of higher temperatures at greater altitudes. Saha found a way out with a flash of insight, that the high-energy spectrum was thanks not to higher temperatures but because the calcium atoms themselves were ionized at higher altitudes.
As ionization consists of separating an electron from the stable configuration of the neutral atom, this requires the use of energy, which again is nothing but raising the gas to higher temperatures. Did Saha’s notion of ionization then amount to the same thing as heating? This is where Saha was able to draw on his familiarity with the dynamics of chemical reactions and work out a way for ionization to be possible even with lower temperatures.
In chemistry, many reactions can take place in either direction, depending on conditions. For example, one reaction is when carbon dioxide reacts with hydrogen to produce carbon monoxide and water. Chemists put it like this:
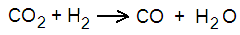
But the opposite is also possible, with carbon monoxide and water vapour giving carbon dioxide and hydrogen, like this:
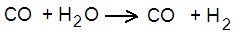
In fact, in this reaction, there is always a mixture of the four components, with both reactions taking place all the time, the extent of progress of one or the other being according to the temperature and pressure.
Saha viewed the ionization of a gas of atoms in the same way. As ionized atoms are just the neutral atoms that have given up an electron, he modeled the thing as a mixture of gases of neutral atoms, ionized atoms and electrons. Neutral atoms kept breaking up into ions and ions kept combining with electrons to become neutral atoms again. Here, Saha used the principles that applied to gases, to the neutral atoms, ions and electrons and managed to get a relation for the equilibrium in terms of the temperature and the pressure. He also introduced, in the calculation, a factor that others had not detailed, the energy required to separate the electron from the atoms during ionization.
The relation he obtained, now known as the Saha Ionisation Formula, showed that ionization decreased with drop in temperature but also increased with drop in pressure. And the level of ionization was greater for atoms than needed less energy to ionize
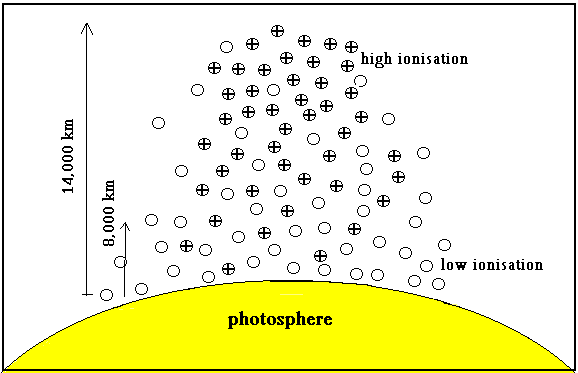
This relation readily gave the level of ionization of calcium at various altitudes in the sun’s atmosphere, where the temperature and pressure could be estimated. At the photosphere, where the temperature is around 7,500º C and the pressure is around our earth’s atmospheric pressure, the formula gives the ionisation at about 34%. But if one goes higher up, where the temperature is only 6000ºC, we find that the pressure has dropped to by a factor of 10,000. This drop in pressure more than compensates for the drop in temperature and the Saha formula gives the ionisation as 95%. This was precisely what is observed in the flash spectrum!
The other question, of why the absorption spectra or some elements are absent at higher altitudes was also explained by the formula. These elements all had very low ionization energies. The result was that these atoms were completely ionized in the chromosphere, leaving no neutral atoms to give rise to the characteristic absorption lines. As for the question of why there was less evidence of hydrogen at high altitudes, this was a bit complex, but again explained by the fact that hydrogen had a high energy of ionisation. A consequence of this was that at higher altitudes, other atoms were ionized and the space was filled with the electrons set free. The relative population of hydrogen atoms thus reduced and they were less prominent. Another paper by Saha, which deals with radiation pressure being selective of the atoms that it pushes up, neatly explained why calcium was abundant at high altitudes. Yes, the Saha Ionisation Formula had developed the mechanism that was operating in the atmosphere of the sun and the stars, which paved the way for further research and discovery!
On the face of it may appear that Saha’s thinking was simple and straightforward, but this simple line of thought was far from obvious in the 1920s. The structure of the atom and the ideas of quantum physics had barely been developed. Astrophysics was not the only area where explanations were wanting. The idea of using statistical methods to deal with a collection of atoms, their temperature, their excitation and their spectra had hardly occurred to anybody. To thread through this maze and arrive at a mathematical device to explain the behaviour of the sun and stars was veritably a triumph.
Saha applied his formula to a host of other elements and came up with results of ionisation levels that were consistently verified. Saha’s ideas got snapped up by researchers the world over and astrophysics came on to the fast track.
In 1923, Saha became professor of Physics at Allahabad University and in 1920 he was elected a Fellow of the Royal Society. At Allahabad, and later as Palit Professor at Kolkata, he continued to do research in forefront areas, like complex spectra, the physics of the atmosphere, river physics and even calendar reform!
When he come to Kolkata in1938 as the Palit Professor, he began to indulge his interest, roused by a trip abroad in 1937, in nuclear physics and set the ball rolling for a cyclotron in Kolkata. He started some work in cosmic ray research in Darjeeling and then he started the Saha Institute of Nuclear Physics, in Kolkata. He then began to associate with public life, economic planning and even served as a Member of Parliament, in the opposition.
He died in 1956, collapsing en route to a meeting in the office of the Planning Commission at New Delhi.
------------------------------------------------------------------------------------------