A roadblock on the way to powerful electric batteries has been cleared, says S.Ananthanarayanan.
The lithium battery is now known to all, as it is used in every cell phone. But this source of power for many everyday devices is in fact a shadow of much better power that is possible with lithium. This is because of the difficulty of handling metallic lithium, one of the most reactive elements known. But a team of scientists at Stanford, California, report in the journal, Nature Nanotechnology, that they have discovered a way to mask the lithium electrode in a lithium battery with nano-particles made of carbon atoms and get around the problem.
The electric battery
A battery is a formation of guns trained on an enemy target and an electric battery is a collection of electric cells acting together. The electric cell is an arrangement of two materials, separated by a medium, where the two materials balance a difference between them by developing electric charges, which can drive an electric current. An example is the simple cell, consisting of a piece of copper and a piece of zinc, with dilute acid between them.

The difference between the two elements, for this purpose, is how strongly the atoms of the elements concerned attract or repel electrons that approach them. Now, atoms consist of a central nucleus, which has all the positive charge and around this are the shells of negatively charged electrons. As these shells act to shield the centre, the nucleus of an atom that has fewer surrounding electrons can be considered to be more exposed and hence more able to attract electrons. So also an atom that has fewer electrons in the outermost shell, the shell that most strongly repels electrons, would attract electrons strongly. On the other hand, atoms with many shells and more electrons in the outer shell, would more effectively keep electrons away.
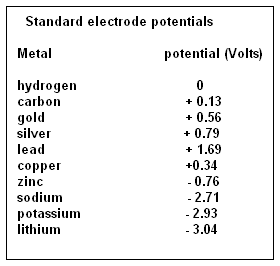
Thus, light alkali metals, which have only one electron in the outer shell, are called electronegative and metals which are heavier and have more electrons in the outer shell, are electropositive. The elements have been ranged, according to an electrical difference, in volts, with reference to hydrogen, as a few shown in the table. We can see that zinc, in the simple cell, is at -0.76 volts and copper is at +0.34 volts. The result is that when placed in a conducting solution, the part of the solution which is rich in electrons collects around the zinc electrode while atoms that have given up an electron crowd around the copper electrode. The two electrodes thus get oppositely charged and if the two electrodes are connected by a wire, a current flows. As the difference, in the tendency to attract electrons is from -0.76 Volts to +0.34 Volts, the voltage of the cell is about 1.1 Volts.
This simple idea of the electric cell has grown, with different materials, like carbon and zinc in the torch cell, or a series of lead electrodes with sulphuric acid, which gets charged when a current is forced through it, and can then discharge, as in the car battery. And with this concept, of storing chemical energy to create electric energy, in electric cells, or of storing electric energy which was generated in a power plant in the form of chemical energy in a storage battery, has grown a universe of electric devices which need to work without a direct connection to a distant power source. Examples are the motor car, the electric torch and of late, the cell phone.
Lithium
A look at the table of electric potential of the elements would show that many pairs of elements could be chosen to form electric cells, and some of them would generate large voltages. Sure enough, many variations of electric cells have been created and have been found practical or otherwise. The combination of zinc and carbon, with semi-solid ammonium chloride in between, has been found practical and has become the standard for simple portable devices. And then there are the nickel-cadmium batteries and the lithium-sulphur batteries.
A look at the table would again show that while the alkali metals, sodium and potassium, have high electronegative potential, the element, lithium is ahead of them all. Sodium has 2.71 Volts, potassium has 2.93 Volts and lithium has 3.01 Volts. Alkali metals are also among the lightest and a piece of lithium floats even in oil. "Of all the materials that one might use … lithium has the greatest potential, says Yi Cui, a professor of Material Science and Engineering at Stanford and leader of the research team. To use the alkali metals in electric cells has thus been the electrical engineers’ dream and would have been realized many times over, but for the fact that the metals are so difficult to handle. Sodium and potassium are highly reactive and need to be kept away from water at all costs. Lithium is not as reactive, but is reactive enough, and inflammable. Its surface corrodes quickly in moist air and lithium needs to be stored under a cover of petroleum jelly. This is the reason that pure lithium has not been successful as an electric cell material. The batteries in use are really lithium ion batteries, with lithium in the material between the electrodes.
Electrodes also tend to expand and deform during charging and use. Graphite and silicon electrodes change volume by a whole 10% or 400% respectively. But the lithium electrode changes volume to a much greater extent. And then, during discharge, when the material of the electrode is deposited back, the deposit is uneven and leads to cracks and fissures, which bring down the efficiency of the cell. And then, the reaction of lithium with the material between the electrodes creates heat, which can lead to fires.
For all these reasons, the authors of the paper in Nature Nanotechnology say, the surface of lithium metal electrode in the battery needs to be covered with a chemically stable and mechanically strong interface. While solutions that have been attempted all fall short of the requirements, the current development of a scaffolding of carbon atoms, in the form of domes which the authors call nano-spheres, to cover the lithium electrode, as shown in the figure, is almost at the ideal.
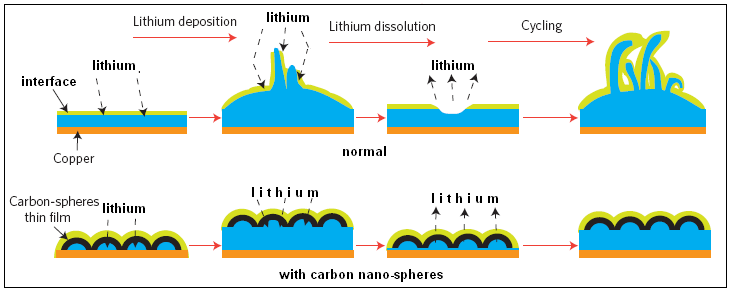
The carbon cover is formed by first coating the copper base with a layer of a plastic material, by covering the base with a solution of plastic particles and then evaporating the solution. Carbon vapour is then deposited on the plastic coat and the carbon atoms to arrange themselves as hexagons. The arrangement is then heated, which creates carbon nano-spheres on the copper surface. The flexible sheet of carbon can be lifted off the copper substrate while lithium is deposited and the carbon nano-sphere sheath makes sure that the lithium deposit does not form filaments. This cover thus protects the lithium surface from deformation during charge and discharge of the battery, while at the same time acting as a chemical separator of the reactive pure lithium from the material in the battery.
¨…..we found a way to protect the lithium from the problems that have plagued it for so long," says Guangyuan Zheng, a doctoral candidate in Cui's lab and first author of the paper. "In practical terms, if we can improve the capacity of batteries to, say, four times today's, that would be exciting." says Stephen Chu, Nobel laureate and former US Secretary of energy, who was part of the research team. As eco-friendly electricity generation develops, more powerful electric batteries would eliminate gasoline use in road transport too.