Getting to know the path of electrons take helps show when they behave as particles and when they do not, says S.Ananthanarayanan.
The interplay of the quantum theory, which says that energy is transferred in discrete ‘lumps’, and the Theory of Relativity, which says matter and energy are equivalent, leads to particles being endowed with wave properties and a to scheme of calculation, called quantum mechanics, which deals with both the manifestations of energy at the same time. But the particles that correspond to waves that we can deal with are so minute, or the waves associated with real life particles are of such short wavelength, that practically observing wave-particle duality has been out of reach.
Xiao-Jing Liu, QuanMiao, Faris Gel’mukhanov, Minna Patanen, Oksana Travnikova, Christophe Nicolas, Hans Ågren, Kiyoshi Ueda and Catalin Miron, working at the Soleil synchrotron, particle accelerator in France, and in Sweden and Japan, describe in the journal, Nature photonics, a method where the two atoms in an oxygen molecule are used as a truly small dimension pair of ‘scatterers’. The action starts when the molecule, on being excited by X Rays, de-excites by emission of an electron from one of the oxygen atoms. The electron emitted behaves like a wave if the electron could have come from either atom, but like a particle, if the atom from which it came could, in principle, be identified. This result casts the die in favour of one side in a celebrated debate between Albert Einstein, and Niels Bohr, one of the fathers of quantum mechanics.
Particle-wave
The distinguishing quality of wave-like behavior is that when waves meet, they can either add and grow stronger, or they can cancel each other out. Light that strikes a pair of slits in a barrier would give rise to a pair of waves arising at each slit. The waves from these two sources striking a screen further on would then create a pattern, called an interference pattern, of bright and dark lines or spots, at places where they add or cancel.
This is the historic Young’s double slit experiment, which laid the basis of the wave theory of light. But now we know that light consists, in fact, of discrete lumps, or particles, called photons. These photons could not have passed through both the slits, as we have imagined, but should have passed through one or the other, and there should be no interference pattern.
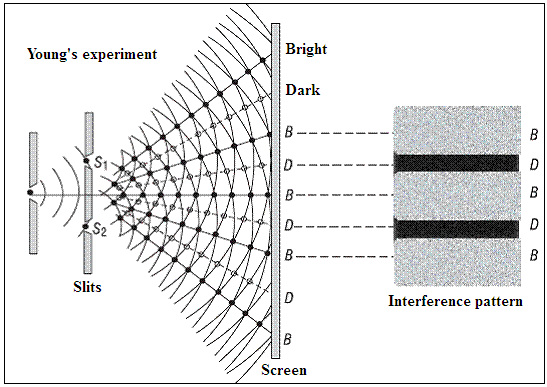
But there is interference nevertheless, because quantum mechanics holds that all things, so long as they are left alone, are in all their possible states of being. This is to say that the photon, if it is left alone, passes through both the slits at the same time, resulting in interference. But if there were a measurement, or one of the slits were closed, then the photon passes only through the slit where it was measured, or the slit that was open, and there is no interference.
Einstein never quite accepted the statistical, or probabilistic method of quantum mechanics, which considered particles to have a spread, or uncertainty of position. In the course of his discussions on wave-particle duality with Niels Bohr, Einstein proposed an imaginary experiment, called a thought experiment, or, in German, a gedankenexperiment, which would show a discrepancy in the idea of duality. The Young’s two slit experiment is considered to work for light because it is not possible to know which slit the photon passed through. Einstein proposed that if the slits were not fixed in the screen, but could move, then the act of the photon passing through one of the other slit would be detectable, in principle, by the recoil of the affected slit. And as the path the photon took could hence be marked by the recoil, there should not have been any interference pattern. The theoretical objection to this was that an act of measuring the recoil accurately this would introduce uncertainty in the position of the slit. This apart, the mass of any real slit in a material screen would make the event of the recoil on the passing of the photon impossible to measure. And if more energetic light of very short wavelength, or a beam of electrons were used, then the slits would have to be too close together to be practical. And for these and other reasons, experimental verification has not been possible and the question remains in the ‘gedanken’.
But the team working at the Soleil synchrotron have found a way to perform the experiment at the molecular level, using the two atoms in oxygen molecules to act as the pair of slits. It is not light that is routed through the atoms, but the experiment is with electrons that are emitted from one of the pair of atoms. If which atom the electron came from is uncertain, then there would be an interference pattern where the electron is detected. But if which atom the electron came from was known, then there would be no interference.
Neutral oxygen molecules are first excited by soft X Rays from the synchrotron radiation. Absorption of X Rays disturbs the electronic linkage of the atoms, which begin to rapidly move apart. The system also de-excites by the emission of an electron. If this emission happens before the atoms separate, the recoil of the electron ejected is shared by the whole molecule and which atom the electron came from cannot be made out. But if the emission happens after the atoms separate, then only the concerned atom recoils. Sensitive measurement of the momenta of the different particles that issue from the interaction enables finding out the whether the recoil has been shared or not, and thus whether the electron came from an identified atom or otherwise.
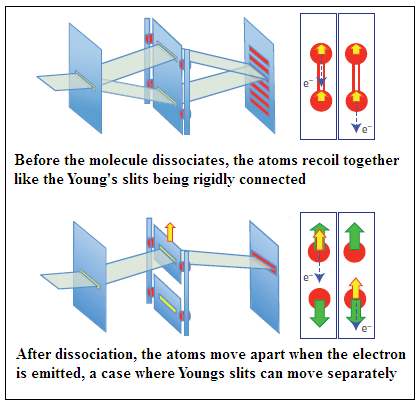
The pattern of detection of the emitted electrons consistently showed an interference pattern every time the emission was from the molecule before the atoms separated, and the interference disappeared when the emission was from a free oxygen ion, after the atoms had separated. The arrangement thus exactly mimics the Young’s slits experiment with light, and also allows for the ‘movable slit’, of the gedankenexperiment of eighty years ago, in the form of the free atom which recoils away from the other atom when the emission of the electron is after the molecule breaks up. When this happens, there is no interference. But if the emission happens earlier, then both atoms recoil together and they cannot be made apart, there is interference, with the electron behaving in a ‘wave-like’ fashion.
Do respond to : response@simplescience.in
------------------------------------------------------------------------------------------