S.Ananthanarayanan reports on a study that represents the coming together of different disciplines, quantum mechanics, statistical mechanics and thermodynamics and the use of supercomputers to enable us to describe the “complex dynamic phenomena taking place inside one of the technologically most used structural materials”.
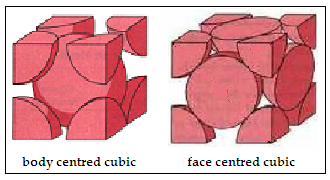
Iron, the material that we find everywhere, from pots and pans to tools and ships, and then to beams and trusses in skyscrapers and bridges, displays peculiar behavior when it is heated. Till it reaches the temperature of 912°C, the crystal structure of metallic iron remains the same, body centred cubic(BCC). But when it crosses that temperature, it changes to face centred cubic (FCC), which is a closer-packed form. This form remains till 1394°C, when it changes back to body centred cubic, till it melts at 1538°C. And there is also a hexagonal close packed structure when iron is under very high pressure.
This apart, iron is magnetic. The magnetic property, of responding to magnetic fields, or to retain magnetism, as a permanent magnet, arises from a property known as spin, of the particles that make up the iron atom. In the atoms of iron, and other metals that display iron-like ferromagnetism, there are odd numbers of electrons, and hence an unpaired electron, the one which results in a net magnetic effect. At lower temperatures, metallic iron forms domains, or regions a few microns across, where the magnetic effects of a group of atoms are aligned, to act like a small magnet. But with mutual interaction, these domains position themselves so that they cancel each other out and a piece of iron normally shows no magnetism
But a further peculiarity is that when iron is heated to 770°C, it loses it magnetic property. There are reasons to hold that it is the magnetic property that favours the low temperature, loosely packed, body centred cubic structure that iron assumes at lower temperatures. But there is clearly more to be said, as iron stays this way till 912°C, even after magnetic behavior is left behind at 770°C.
Mauger L, Lucas MS, Muñoz JA, Tracy SJ Kresch M, Xiao Yumin, Chow Paul and Fultz B, of Caltech, Air Force research lab, Ohio, from California and the Carnegie Institute of Washington at Illinois, report in the journal, Physical Review B, that their study of the behavior of the mechanical vibration and order and disorder in magnetic alignment in metallic iron, as the temperature changes, indicates correlation between the energy stored in the two reservoirs. The finding has allowed the researchers to conclude, for the first time, that it is this interaction at moderate temperatures that allows the body centred cubic structure of iron to remain even after the alignment of magnetic elements has disappeared.
The finding also agrees with the results of theoretical calculation, made by Mauger, Fultz and others, with Jörg Neugebauer, at his laboratory in the Max Planck Institute at Düsseldorf, thus validating the computation carried out.
Thermodynamics
The way systems of particles, like solids, liquids or gases behave depends on the way the total energy of the system is distributed. The simple gas laws, for instance, are explained by statistical models of molecules of gas bouncing off each other and the walls of the container. This is also true of liquids, where molecules, which are the particles, hold on to each other in a lower energy state, but are still able to flow and take the shape of a container. And the masses and other properties of the molecules dictate when this stability will break down and change the state into that of a gas. At lower energies still, we have the solid state, where the atoms constituting the material are in fixed positions, held together by electrical forces, and sometimes, magnetic forces. The energy of the system shows itself in motion of the atoms or groups of atoms, generally of vibration, like masses supported by springs that stretch and compress. And different crystal structures, each of which may be the most stable at different levels of energy, may be favoured at different temperatures.
The water molecule, for instance, forms different forms of ice as it warms to the melting point. A feature of the water molecule is that the two hydrogen atoms are not symmetrically placed on either side of the one oxygen atom. This makes the molecule behave like a pair of charges at moderate distances and this results in complicated changes in crystal structure as temperature varies. This feature even causes structure to dominate the liquid state just after melting, with shrinking, rather than expansion, as water warms from freezing, at 0°C to 4°C.
In iron also, on account of ferromagnetism, the energy of an assemblage of atoms has energy of magnetic linkage, in addition to the electrical forces that keep the crystal together, along with the mechanical energy of lattice vibrations. It would appear that this is the reason that iron exhibits diversity and anomaly in its disposition as the temperature changes.
The Caltech group assessed the actual nature of vibration energy in iron, as the temperature is raised from to the point where magnetism is lost and then to the point where the crystal structure changes from BCC to FCC. As the particles that are vibrating are individual atoms and the distances are at the scale of the inter-atomic, the dynamics follows the rules of quantum mechanics. The vibrations move as very high frequency waves within the crystals and the waves have a particle character, and manifest as phonons, just like light waves move in packets that are known as photons. Energy stored in the alignment of magnetic moments also shows itself as waves, to appear as particles, known as magnons, and there is theoretical computation of their behavior.
The Caltech group assessed the vibration modes present in iron by scattering of specific X Ray frequencies by the phonons. The X Rays were generated in the particle accelerator at Argonne National Laboratory in Argonne, Illinois. This facility works on the principle that accelerated charges emit light of frequency that depends on the acceleration. Thus, by varying the energy of the particles in the accelerator, it is possible to obtain X Ray emission at frequencies of choice.
When the vibration measurements that were recorded were considered along with the known magnetic behavior of iron at the different temperatures, it was found that energy stored in vibration was more than expected, with the excess, arising out of frequencies which were not harmonics of characterisic frequencies, corresponding to the levels of energy stored in magnetic effects. This suggests that magnetic effects and lattice vibrations act together at moderate temperatures. At higher temperatures, the force between atoms in the lattice were seen to grow weaker, which allows the BCC structure to continue after magnetic effects are lost due to higher energies. This would also correspond to the loss of tensile strength of iron and steel at higher temperature.
The work represents the coming together of different disciplines, quantum mechanics, statistical mechanics and thermodynamics and the use of supercomputers to enable us “to describe the complex dynamic phenomena taking place inside one of the technologically most used structural materials," says Neugebauer, of Düsseldorf . The development of grades of iron and steel, over the centuries, has been largely ‘trial and error’. "The newly gained insight of how thermodynamic stability is realized in iron will help to make the design of new steels more systematic,” Neugebauer said.
Steel frame of World Trade Centre
The collapse of the steel frame of the Twin Towers, WTC, New York came as a surprise to many, as why should a fire in the upper floors cause the building to collapse? One reason given was that the fire caused the steel frame to melt. But this was discarded as the temperature of burning gasoline is not so high. But then, the steel really did not need to melt to become incapable of supporting the load. The temperature in some of the higher floors got high enough to reduce the strength of the steel, though well below its melting point, and those floors collapsed. The impact of falling steel and concrete then communicated to the lower floors, which also collapsed.

------------------------------------------------------------------------------------------