Imitating nature’s trick may check the earth’s greatest pollutant as soon as it is produced, says S.Ananthanarayanan.
The earth’s living things owe themselves to the oxygen in the atmosphere. And the earth is just right for them to thrive thanks to carbon dioxide in the atmosphere being in check. And both these qualities of the earth are thanks to plants and organisms that use the sun’s energy to turn CO2 into food and oxygen. But humankind is producing more CO2 than can be taken care of and the build-up is disturbing the balance of the planet. Locking up all the CO2, so that it does not get out there, may not be viable. Can we create machines that convert CO2 like plants or bacteria do, so that it ceases to be a menace?
Peidong Yang, Christopher Chang and Michelle Chang, working with Chong Liu, Joseph Gallagher, Kelsey Sakimoto and Eva Nichols, at the U.S. Department of Energy’s Lawrence Berkeley National Laboratory and the University of California at Berkeley report in the journal, Nano Letters, that they have found a way to do just that - a device, consisting of nano-wires and bacteria, that mimics the process of photosynthesis, by which plants use the energy in sunlight to synthesize carbohydrates from carbon dioxide and water.
Photosynthesis could be regarded as the ideal in conversion systems, where the raw material, carbon dioxide and water , is dismantled into components and reassembled as carbohydrates and free oxygen, directly using photons of light as the energy source. There are no undesired by-products, no heat, no electricity. And around the centres of photosynthesis, a framework has evolved for the most efficient collection of light.
We can readily see that carbon combining with oxygen, which happens when things burn, is a case of oxidation, and this releases heat. Photosynthesis is the opposite, where carbon in carbon dioxide is, in effect, separated from oxygen, through a reaction called reduction, and which consumes energy. The reduction is carried out by adding hydrogen to CO2, to form carbohydrates, and in photosynthesis, as carried out by plants and bacteria, the hydrogen comes from splitting water molecules into hydrogen and oxygen. In the water molecule, hydrogen atoms give up their electrons to oxygen atoms, to form a stable structure. When water is split, by the action of photons, oxygen atoms get together as oxygen molecules and electrons, and energy, become available to ‘fix’ CO2 as carbohydrates. The effect of photosynthesis is thus to store the energy of photons, partly in carbohydrates and partly in free oxygen. It is this energy that is given up when free oxygen meets carbon, either in a coal fire or when carbohydrates, like glucose, fuel the action within the bodies of living things.
The actual photon capture and splitting of water molecules is carried out in plants mainly by the pigment chlorophyll. Each chlorophyll molecule captures one photon and loses an electron, which moves through a chain of other chemical agents, as a form of energy currency. And while this action proceeds, the chlorophyll molecule regains its lost electron from water molecules, to release oxygen, which is, in fact, is a ‘waste’ product of the process.
Along with this process of using the photon energy, is the system for the most efficient capture and use of the photons themselves. In plant cells the chlorophyll molecules are distributed over folded membranes which contain structures called chloroplasts. A cell can have 10 to 100 chloroplasts and leaf tissue can have 450,000 to 800,000 chloroplasts in every square millimeter. And within the cells, the light sensitive molecules are arranged in a complex like an antenna, act together to photons with quantum mechanical efficiency.
Artificial photosynthesis
The arrangement of the Berkley group mimics nature’s method by using a mesh of nano-wires made of silicon and titanium oxide, to act as the photon capture and electron source, and microbes that speed up the reduction of CO2 to useful organic chemicals. When photons strike the silicon surface, loosely bound electrons are ejected, and used to feed the bacterium Sporomusa ovata which readily accepts electrons directly from the surrounding environment and uses them to reduce carbon dioxide. The ejection of electrons is matched by generation of ‘holes’ or the ‘lack of electrons’ in the titanium oxide, which acts on water to generate hydrogen and release oxygen.
The use of nano-wires ensures the high surface area for efficient light gathering. The ‘artificial forest’ of nano-wires “is similar to the chloroplasts in green plants,” says Peidong Yang, chemist with Berkeley Lab’s Materials Sciences Division. Many bacteria are anaerobic, and oxygen sensitive, but embedded within the nano-wire mesh, they are able to work in an oxygen free environment, such as of flue gases. In the ‘proof of principle’ trial by the Berkley group, however, the bacterium, S. ovata was able to act under aerobic conditions, which is 21% oxygen, with good electrical efficiency stability. "S. ovata is a great carbon dioxide catalyst as it makes acetate, a versatile chemical intermediate that can be used to manufacture a diverse array of useful chemicals," says Michelle Chang, synthetic biologist and one of the corresponding authors of the paper. "We were able to uniformly populate our nano-wire array with S. ovata using buffered brackish water with trace vitamins as the only organic component."
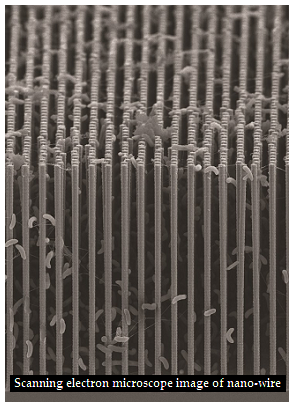
The trials, which represent an alliance between material science and biology, manages to create the key elements of the nano-wire light capturing mechanism, and the CO2 conversion by catalytic activity of the bacteria. The arrangement has been able to convert solar energy with an efficiency of 0.38%, continuously for 200 hours under simulated sunlight. This is about the same as the efficiency of a natural leaf
“We are currently working on our second generation system which has a solar-to-chemical conversion efficiency of three-percent,” Yang says. “Once we can reach a conversion efficiency of 10-percent in a cost effective manner, the technology should be commercially viable.” Once the CO2 has been converted to acetate, or other form, genetic engineered e.coli can be employed to generate specific chemical products. During the trials, the performance of conversion was 26% for butanol, a fuel comparable to gasoline, 25% for, a precursor to an anti-malaria drug, and 52% for PHB, the renewable and biodegradable plastic. Artificial photosynthesis thus has the potential of treating large CO2 output of facilities like power plants by using only water and sunlight to produce chemicals – a doubly green chemical industry!
------------------------------------------------------------------------------------------