The method that bacteria use to keep viruses in their place will now help humans tidy the human genome, says S.Ananthanarayanan.
The human genome is library of over 20,000 genes, which code for components of some 2 million proteins, and are located over the length of the DNA, which has over 3 billion basic units. This genetic blueprint, which we carry in each of our 100 trillion cells, is a complex document and, in some parts, it directs the body to disease instead of health. We now know how to detect these problem genes, but doing anything about it is a different matter.
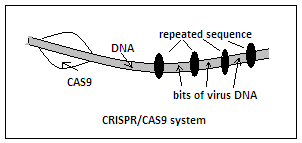
The discovery started with the observation that the chromosome, which is mostly DNA, of many bacteria contains a series of sequences that are repeated, interspersed with sequences that are derived from viruses. These repeats in the bacterial DNA, however, did not make much sense, till it was noticed that they usually occurred in the vicinity of another set of genes, of a kind that seemed to be involved in the process of DNA repair. It was then thought that the repeats were perhaps some kind of template acquired from encounters with viruses and later used for resisting the same viruses. The repeats were named, CRISPR, a short form for Clusters of Regularly Interspaced Short Palindromic Repeats, and the associated genes were called CAS genes, for CRISPR Associated genes.
While the DNA are the construction formula of all the proteins that the organism would need, the whole DNA is never active at once. Instead, it is portions of the DNA, that represent the code for just a set of proteins, that are extracted and utilised at each occasion of expression of genes and the creation of the different proteins that define the nature of a cell. The medium to transmit the partial code to the cellsí engines that carry out the protein formation is the RNA, a variation of the DNA molecule structure. Now, when a virus attacks a bacterium, the identifying sequences of the virus get copied on to scraps of RNA which form during the encounter with the virus and these get stored in the CRISPR sequences. When the virus attacks again, it is not a passive action of taking and storing copies of parts of the virus DNA that happens, but the existing patterns of the virus DNA are copied quickly on to RNA, which then combine with proteins, or enzymes, that are formed from the CAS genes portion, enzymes of which an important group have been called CAS9.
CAS9, equipped with the template for a portion of the virus, is then able to zero in to just the portion of the virus DNA that CRISPR has recorded and CAS9 does its wonderful thing of snipping the DNA just at that place. The virus, which consists mainly of its own DNA, is then destroyed and the bacterium stays healthy.
In 2012, scientist Jennifer Doudna, of the University of California at Berkley, and her colleague Emmanuelle Carpentier, and their team, studied the process of this action and they identified CAS9 as the enzyme which could cause a rupture in the two strands of the DNA molecule at the portion marked by the pattern of the RNA, called the guide RNA. When they had got down to how this happened in the bacteria, they wondered if the process could be mimicked with a given RNA to serve as the guide RNA, to create a system that could sever the DNA at the place specified by the guide RNA that they had used.
The team went on to do just this and then they devised a simple test to see if the target DNA had actually been cloven at the point they wanted. They first generated a pair of guide RNA that matched specific parts of a known DNA molecule and then formed the cleaving enzymes by letting the RNA attach to CAS9. The target DNA was then incubated with the cleaving enzymes, and then, the DNA was examined with the help of a gel in which segments of DNA move with different speeds. The gel was able to separate two parts of the DNA and the sizes of the portions showed that the original DNA had been cut just where the cleavage was programmed by the guide RNA.
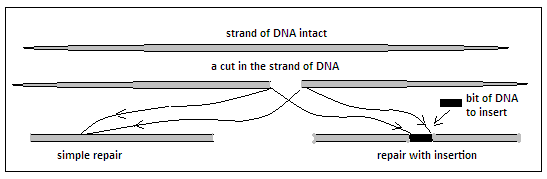
This discovery, Jennifer Doudna says, was exciting, as studies had shown for some time that cells had ways of repairing DNA damage. In such cases, when a DNA was separated, the two portions rejoined, either just as they had broken or with a small section added, or there could be the addition of a whole part of DNA which matched the two broken ends. Where the DNA rejoined in the ordinary way, there was often damage to the gene involved, which had its value in studying the properties or function of that gene. Where a portion was added, the genome now had additional genes, with their value in research. These features of DNA repair tempted scientists to hope for a way to break DNA at a place where there was an interest for repair, so that introduction of a predetermined section could become possible. The CRISPR and CAS9 system amounted to just that, that the DNA molecules could be divided accurately at the place where there was interest in carrying out a modification.
After the nature of the DNA, as a chain built up of just four kind of links, groups of atoms called A, G, T and C, in sequences that code for the production of all the proteins the body needs, was worked out by Crick and Watson in 1953, methods have been devised to navigate over the length of the chain and agents that are able to affect specific parts of the chain have been developed, etc. Specific genes that are implicated in specific diseases have been identified and there are methods to breed animals that lack that gene, or have a variation, to check whether this leads to remission of symptoms. The field is one of great promise, to treat genetic disease, generate crop varieties with greater yield or resistance to pests, bacteria that can change waste into useful substances, etc, but the processes to make modifications in the genome are painstaking and time consuming.
In this context, the CRISPR/CAS9 system, which provides the scientist and the industry the means to intervene directly at the DNA level, takes the possibilities of genetic engineering to another plane. It is now feasible to modify the genome of just any creature to any extent, within a reasonably short period of time. This has its own dangers if used without care, but it could also enable bringing about very fast ecological changes to help the world cope with the challenges of warming in the coming decades.
------------------------------------------------------------------------------------------