Finding new antibiotics as fast as bacteria evade existing ones has been an unequal fight, says S.Ananthanarayanan.
Resistant bacteria and the growth of ‘super bugs’ could convert even a simple surgery into a high risk procedure and common infections could become as dangerous as they were before the discovery of antibiotics. A study in the UK has estimated that by 2050, antibiotic resistant bacteria could kill one person every three seconds and cost the world trillions of dollars in economic loss.
Apart from uncontrolled use of antibiotics, the higher human population and increased communications which help resistant strains to spread, have led to exponential multiplication of new bacterial forms. The speed with which bacteria are able to evolve has begun to outstrip the capacity of pharmaceutical research to come up with new drugs. In the context, a method to quickly assemble antibiotic molecules from basic constituents, which has been described in the journal, Nature, may be the answer to the grim prospect of bacteria becoming progressively immune.
Ian B. Seiple, Ziyang Zhang, Pavol Jakubec, Audrey Langlois-Mercier, Peter M. Wright, Daniel T. Hog, Kazuo Yabu, Senkara Rao Allu, Takehiro Fukuzaki, Peter N. Carlsen, Yoshiaki Kitamura, Xiang Zhou, Matthew L. Condakes, Filip T. Szczypinski, William D. Green and Andrew G. Myers, at Harvard University, Massachusetts, report in their paper that they have built over 300 different molecules with antibiotic activity, including some that are akin to the antibiotic, erythromycin, starting from basic components. Some of the variations are even active against bacteria that are resistant to existing antibiotics, the paper says.
Antibiotics
The era of antibiotics started with the identification of penicillin by Alexander Fleming in 1928. Fleming noticed that bacterial cultures were killed or their growth impeded by an accidental scrap of a fungal growth, or a mould. Fleming was then able to show that the mouldy material was effective against a number of bacteria. This explained the use of mouldy bread, for instance, to help wounds heal, but Isolating the active agent, which was called penicillin, was challenging. It was only after the efforts by Howard Florey and others in 1939, that penicillin could be used as a drug and research efforts during World War II led to methods of large scale production. A number of other penicillin-like antibacterial substances have since been discovered and antibiotics are now the mainstay of surgeons and physicians.
The way antibiotics act is that their complex molecular structure has portions that are able to attach to specific parts of the exterior of a bacterium, and hence to suppress its life processes or impede its action. Specific antibiotics are then able to deal with specific pathogens or a class of bacteria without serious effects on the body processes of the infected person. The actual antibiotic molecules, however, are too complex to fabricate and need to be formed by bacterial action or fermentation of complex, biological starting materials, a process known as semisynthesis. Modifications are still necessary for the substance to have the desired antibacterial effect, and these are carried out using chemical means. The process is painstaking and involves first treating the molecule so that its major portions are protected, carrying out the change in the target portion and then uncovering the protected parts. Manufacture in quantity, after this, is again through biological processes.
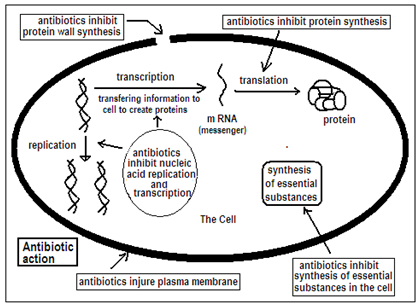
Just as antibiotics need special structural features to be useful, bacteria are also sensitive to antibiotics on account of their own specific surface features. Hence, if a bacterium evolves, by chance mutation, to change those specific surface features, the strain of the bacterium could be as effective in causing disease as before, but immune to some antibiotics. This is the mechanism by which resistant strains of bacteria arise and indiscriminate use of antibiotics in low doses can result in the resistant proportion dominating the population.
Scientists then need to get busy trying to make changes in the structure of available antibiotics to restore effectiveness against the bacterium. This has generally been possible, using sophisticated methods to identify the changes needed and then the complex chemical procedure. In this way, with many trials and experimentation, a new antibiotic to deal with the resistant strain can be developed from the previous version of the antibiotic.
With many rounds of such modification, however, and also rapid changes in the features of bacteria, this process has started getting more difficult or the changes required are not feasible. The rise in human population brings about crowding of hosts and greater mobility of bacteria. International travel and trade also facilitates the transport of bacteria and a resistant strain is easily able to spread over a wide area and establish itself. The numbers of resistant strains of bacteria have thus started increasing. 480,000 new cases of multi-drug-resistant tuberculosis were reported in 2013. Extensively drug-resistant tuberculosis, which needs longer and less effective treatment, has been identified in 100 countries. And the trend with many other infections and diseases is the same. The World Health Organisation has hence approved an emergency global plan to combat antibiotic resistance and the bleak forecast for 2050 put out by the UK based study.
Antibiotic assembly
The Harvard University researchers have gone about creating modified antibiotics by a different route. They took on the synthesis of a group of antibiotics called Macrolides – whose structure includes the large ring in the left half of the Erythromycin molecule, which is shown in the picture. To this ring are attached different chemical groups, to give rise to different Macrolides, a section of which are antibiotics. Building this structure in the laboratory has not been feasible, and the route to macrolide antibiotics has been only through biological templates.
What the Harvard researchers did was to split the Macrolide molecule into eight different modules, or building blocks, as shown by the different colours in the picture, and undertook only the task of synthesizing the simple modules. When dealing with simpler modules to create the Macrolide ring, it is also relatively easy to attach the different chemical groups that we may like to have in the final molecules. When the building blocks were ready, the researchers carried out seven key coupling maneuvers to progressively link the modules together, as shown in the picture. This process sidestepped the difficulties in the conventional way of building the Macrolide molecule, where most attempts failed while creating the ring structure.
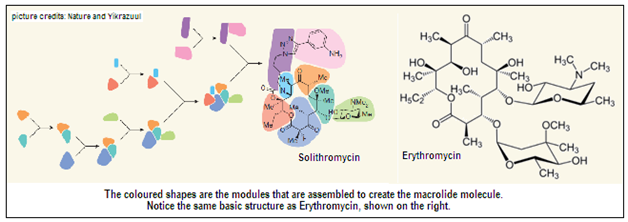
The variety of chemical groups that can be attached to the separate portions of the final molecule, and also the process of linking the parts together enable a very high degree of variations. The Harvard group was hence able to create over 300 variations of the Macrolide structure, as candidates for development as useful antibiotics. One was the antibiotic, Solithromycin, which is currently produced by carrying out sixteen modifications to Erythromycin, the papers says. 305 of the Macrolides created were tested against a panel of pathogens, including well known and notorious antibiotic resistant bacteria. Most of the Macrolides showed promising potency, the paper says.
The work done is hence a platform for creating an unequalled variety, with simple permutations of the components of Macrolide antibiotics, for experimentation and synthesis if found useful. There would be need for more study of side effects and effectiveness of these Macrolides in use as a drug, but the industry now has a starting point that is most of the way to the finishing line, the paper says. Finding similar ways to synthesise other naturally occurring antibiotic families would be a logical sequel, the paper adds.
------------------------------------------------------------------------------------------