The protective armour that saves the brain from harmful things also prevents entry of curative agents, says S.Ananthanarayanan.
The blood vessels that serve the brain are lined with closely packed cells that prevent all but gases, like oxygen and vital things, like water and glucose, from getting into the brain. The trouble is that this barrier also keeps out any drugs that need to get there when there is injury. The current treatment of brain injury or other disease is hence limited to passive maintenance or invasive therapy that has its own drawbacks.
Aman P. Mann, Pablo Scodeller, Sazid Hussain, Jinmyoung Joo, Ester Kwon, Gary B. Braun, Tarmo Molder, Zhi-Gang She, Venkata Ramana Kotamraju, Barbara Ranscht, Stan Krajewski, Tambet Teesalu, Sangeeta Bhatia, Michael J. Sailor and Erkki Ruoslahti, from different institutes of science and medicine in California, Massachusetts and in Estonia report in the journal, Nature Communications that they have developed a method of getting drugs through the shield and right where they are needed. They report that they have found a molecular sequence that zeroes in just where the brain has been injured and could thus form the vehicle to transport drugs that could limit the damage

The brain needs a huge supply of energy and oxygen to keep working and the body provides this by generous blood supply. The adult brain gets about three quarters of a litre of blood every minute and this is 15% of all the blood that the heart pumps out. Such a large consumer of blood also needs blood that is reasonably free from impurities and toxins. As the function of the blood is to move to and from the whole body a variety of substances, including wastes, the blood ends up with a load of larger molecules, which include toxins and bacteria. The blood vessels in most parts of the brain are thus provided with a lining that only allows oxygen, water and essential nutrients, like glucose and some amino acids to diffuse through and does not allow normal exit and entry that is possible through blood vessels in other parts of the body.
This feature, which separates the circulating blood from the fluid around the cells of the brain, is called the blood-brain barrier and protects the brain from most pathogens. Infections of the brain that come through the blood are thus very rare. Antibodies that the immune system creates are too large to cross the blood–brain barrier and only certain antibiotics are able to pass. The few infections that do occur are hence difficult to treat. In some cases, drugs are injected directly into the cerebrospinal fluid, where they can enter the brain, but this is neither possible nor effective most of the time. The procedure is also risky as it amounts to physically violating the security arrangement that has been set up specifically to keep toxins out.
There is hence no way to actively treat acute brain injury, the authors of the Nature Communications paper say, and the prognosis is poor. Traumatic Brain Injury, as such injury is known, the authors say, is termed a ‘silent epidemic’, and it is a major cause of disease and death in children, teens and active adults till the age of 44. In certain cases, they say, disease or injury does cause breakdown of the blood-brain barrier, which could allow entry of large molecules or drugs via the blood circulation. The entry alone of drugs, however, is not good enough as they diffuse and do not stay at the place of injury, the authors say.
Marking the site of injury
As a way to overcome this problem of getting the therapeutic agent to the site of damage, even when the blood-brain barrier had been pierced, the authors recall that they had earlier used a method to detect specific molecular signatures at places of injury to tissue. The way cells, proteins and viruses communicate is with the help of indentations on their surfaces. Proteins are long sequences of several of the twenty types of building blocks, called amino acids, and each one has a unique structure. A virus infected with the coding for a short length of protein would hence display the same specific pattern, and attach to only those cells, if any, which have the structure that matches the pattern of the amino acid sequence that the virus expresses. If there is a library of viruses treated with different amino acid sequences, the pattern that corresponds to the cells affected by a particular virus can then be found out.
Using this method, the researchers had found that many injury sites displayed affinity to specific amino acid sequences. The same thing should be possible at the site of injury within the brain, where specific changes in the molecular environment had been observed, they reasoned. Accordingly, they set out to see what chain of amino acids, or scrap of protein, may correspond to the molecular changes at the site of brain injury, so that a means of using this information to direct drug delivery could be devised
The method used was to create puncture wounds to the right hemisphere of the brains of experimental, adult, male mice. This caused rupture of the blood-brain barrier and antibodies in the blood leaked into the mass of brain tissue. Six hours later, a library of probe viruses marked with different scraps of amino acid sequences was injected into the blood stream of the mice. Thirty minutes later, when the extent of viruses present in the brain was assessed, it was found to be mostly in the right hemisphere, showing that it was there that the barrier had been pierced. And of the kind of viruses present, it was found that the ones marked with a particular four-amino acid sequence – CAQK – for Cysteine, Alanine, Glutamine (denoted as ‘Q’) and Lysine (denoted as ‘K’), were the ones that had remained at the injury site. As CAQK appeared to seek out and remain at the injury site, it looked like CAQK could be used to guide therapeutic substances.
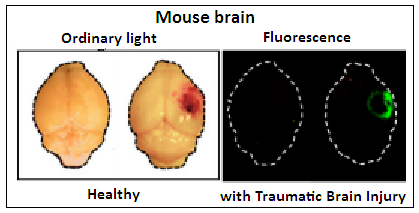
A control test, using CAQK amino acid chain that had been marked with a fluorescent label was injected, and it was found that the injured part of the brain lit up with fluorescence. Further tests, with injury to other organs, like the liver, or the skin, showed that homing of CAQK was specific to brain injury. CAQK homing was seen to be there even five days after the injury , which suggests that CAQK could be used to target the place of injury for a period. And it was seen that accumulation of CAQK remained in place a good three hours after the injection.
Further trials using silver nanoparticles that had been treated with CAQK also showed that the nanoparticles accumulated at the injury site, just like the virus probes. Payloads of bits of DNA were also guided to the injury spot in the brain, with no accumulation at other, normal areas. And finally, testing with sections of tissue from human brains showed that CAQK behaved in the same way with injured human brain tissue too. “These findings present an effective targeting strategy for the delivery of therapeutics in clinical management of acute brain injuries,” the authors say in the paper.
Traumatic Brain Injuries are estimated to cost $ 76.5 billion a year. More than 1.7 million are believed to occur each year and 75% of them are concussion. 1.4 million are emergencies, 2.75,000 need hospitalization and 52,000 result in death. The common causes are falls (35%), car accidents (17%), collisions (17%) and assaults (10%)
------------------------------------------------------------------------------------------