Some paints used by master artists discolour with , says S.Ananthanarayanan.
Gradual changes in the bright yellow flowers in Vincent Van Gogh’s 1887 paining, “Flowers in a blue vase” to an orange-grey colour, have caused grave concern to the Kröller-Müller Museum, which acquired the painting early in the 20th Century. The cadmium sulphide paint that Van Gogh had used is known to oxidize in the air, getting covered by a slightly off-white, transparent layer and losing colour and luminosity. But what was seen was an orange-grey crust, which could not be removed without damaging the original paint.

The journal, Analytical Chemistry, carried a report by scientists of Antwerp, Delft University of Technology and scientists from France and Holland, of high energy X Ray study of the surface of the painting. The study revealed the nature of the colour changes as being a degradation process at the interface of the cadmium paint and the layer of varnish that is there to protect the paint. The sources of high energy X Ray were the European Synchrotron Radiation Facility (ESRF) at Grenoble, France and the Deutsches Elektronen-Synchrotron (DESY) at Hamburg
Paint and pigments
Modern oil paints are believed to have been formally invented in the early 15th century by the Flemish painter, Jan van Eyck, who mixed mineral pigments with an oil, most often linseed oil, that would gradually dry and harden. A series of painting masters perfected the method of mixing minerals and oils, or even beeswax. Artists used to grind their own pigments and carefully mix in the oils in the correct proportions. Modern paints use a number of plant based oils, adjusted for viscosity and modern manufacture ensures consistency, while delivery in tubes even helped in a method of application on the canvas.Paint gets its colour from small particles of pigment that are suspended in the oily carrier. Mineral oxides, like lead oxide, now replaced by zinc or titanium, give white paint, while cadmium, copper, arsenic, mercury, iron, cobalt, chromium, not just as oxides but in combination with sulpur or carbon, are used for different pastels. Combination of pigments produces a range of shades, while the artist mixes colours on his palette for the final effect. But the colours are basically because of the different coloured salts – and these salts can be affected by heat, humidity and the gases in the air.
Cadmium paints
Cadmium is a silvery white metal but the colour of salts of cadmium can be red, yellow or green. Cadmium pigments are usually yellow, orange or red and about half the cadmium produced worldwide is used for making paint, although its use is declining, as cadmium is poisonous. But in the late 19th century cadmium paints were a newly discovered medium and were widely used by artists. The cadmium sulphide, yellow pigment used by Van Gogh was one such.Cadmium sulphide is known to get oxidized, to cadmium suphate, which is dull yellow. Paintings were hence coated with a layer of transparent varnish, to protect the pigments. Van Gogh, himself, produced all his 800 paintings and 700 drawings within 10 short years (till he died, in 1890 at the age of 37) and did not cover any of his painting works with varnish. But with cadmium paints found to discolour, most Van Goghs were covered in varnish in the early 20th century and the Kröller-Müller Museum did the same with Van Gogh’s ‘Flowers in a blue vase’, which they had acquired
This is the context of the discovery in 2009 that even under the coat of varnish, the yellow flowers in cadmium paint had turned darker. "The removal of the orange-grey crust and discoloured varnish was not possible without affecting the very fragile original cadmium yellow paint on these parts," paintings conservator Margje Leeuwestein from the Kröller-Müller Museum says. As ordinary methods did not seem feasible, the museum extracted two microscopic paint samples from the affected parts of the painting and sent them to Koen Janssens from the University of Antwerp for analysis.
X Ray studyThe changes in the nature of the coat on the canvas were not at the level of particles or specks of pigment, that any microscopic analysis was possible. The changes are at the atom level and analysis required probing by X Ray.s of short and controlled wavelength. X Ray beams are scattered by individual atoms and the scattering pattern reveals how the atoms are oriented and what atoms they are – ie, the internal, atomic structure of the grey-brown crust at the place where the cadmium paint and the varnish made contact.
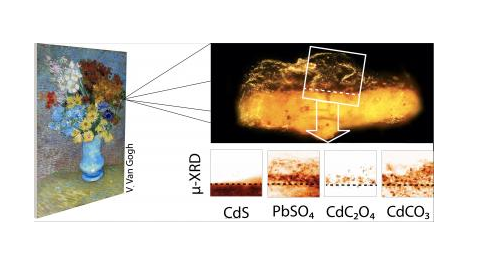
The scientists were surprised to find that even if the cadmium sulphide had oxidized, no crystals of cadmium sulphate, or its compounds were present. But, "it emerged that the sulphate anions had found a suitable reaction partner in lead ions from the varnish and had formed anglesite (lead sulphate)," says DESY scientist Gerald Falkenberg. Anglesite is an opaque compound that was found nearly everywhere throughout the varnish. The sulphate had arisen from the cadmium sulphide pigment and "the source of the lead probably is a lead-based siccative (thickening agent) that had been added to the varnish," adds Falkenberg.
"The research into this hitherto unknown degradation process of varnished cadmium yellow oil paint allows to better understand the current appearance of the painting," explains Leeuwestein. Joris Dik from TU Delft adds that "it also provides information on how later-applied varnish layers may contribute to the decline of certain pigments of a painting. In the future, this degradation process can hopefully be inhibited or even prevented thanks to novel preservation and conservation techniques." Whether removing the varnish and crusts from paintings with this type of degradation is possible and appropriate is not yet fully understood. Leeuwestein adds that "in every similar case of a possible varnish and crust removal, it should always be considered that this varnish and crust contain original material from the cadmium yellow oil paint. The possible removal of original material from a painting during a conservation treatment is of course undesirable."
"Many of Van Gogh's French period paintings have been inappropriately varnished in the past and removal of these non-original varnish layers is one of the challenges facing conservators on a world-wide basis today. The type of information provided by Janssens and his team is vital to support the difficult decisions that conservators often have to make regarding such complex cleaning treatments," says Ella Hendricks, Head of Conservation of the Van Gogh Museum in Amsterdam.
------------------------------------------------------------------------------------------ Do respond to : response@simplescience.in