Hydrogen energy is clean, but how about production, asks S.Ananthanarayanan.
While the world hesitates to buckle down and use less energy, the quest is on find the energy we need without the carbon that has always gone with it.
While coal and petroleum are still the main sources, and many questions remain about nuclear power, wind and solar have become important alternatives. These, however, being intermittent, could do with technology to store power till it is needed. While electricity, where it is produced with low carbon, has become the green propellant in many areas, the storage battery brings in a dark, brown streak. In the context, hydrogen gas is emerging as an acceptable, low pollution intermediary in the transfer of energy.
The hydrogen-based fuel cell, which generates electricity, is being fast adapted for practical uses, and hydrogen has come forward as a light-weight container of energy. While there is a group that has devised a low-cost use of hydrogen to turn CO2 into aviation fuel, there is world-wide interest in hydrogen use, and ways to generate hydrogen with the lightest carbon footprint.
The world’s stock of energy is essentially chemical energy, in the form of coal or hydrocarbons, which have accumulated solar energy, captured by photosynthesis, over millennia. We then extract the energy by burning the fuel, to drive the steam engine, the petrol engine, or using these engines, the electric generator. There is hence conversion of chemical energy to mechanical energy, and then to electricity. Electricity is then transmitted, to be available where required, usually to be converted to mechanical energy. Needless to say, the CO2 goes back into the atmosphere.
The fuel cell reduces a step in this sequence, by converting chemical energy directly into electricity. While this would eliminate the wastage that arises because each conversion has limited efficiency, the use of hydrogen in the cell also eliminates the emission of CO2 at the stage of the cell.
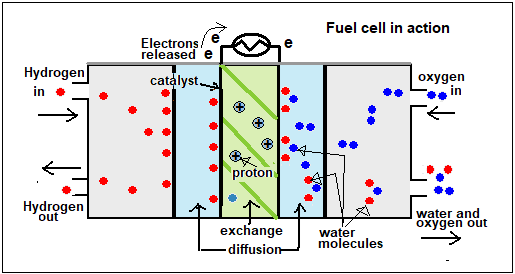
The principle of electrical cells is that energy is released when chemicals change from one form to another. While this energy could show as ‘heat released by the conversion’, it can also be tapped and used to drive an electric current. In the hydrogen fuel cell, the energy used is what comes off when hydrogen combines with oxygen, to form water. Normally, this could be explosive, or it could be controlled, as in a flame. What happens in the fuel cell, however, is that the hydrogen fuel is stripped of electrons, with the help of a catalyst, and the electrons travel by a separate path, to re-join the hydrogen and oxygen where they get together, as water.
The fuel cell can thus generate electricity as long as the hydrogen and oxygen, or other fuel substances, are there. The first fuel cell was invented in 1838, and was resurrected, in the hydrogen-oxygen form, in 1932. The hydrogen-oxygen fuel cell now has many applications, a notable one being aboard satellites and space capsules. And even on the ground, the fuel cell electric car runs the same 300 kms on a tank of hydrogen, like a conventional car, but twice as efficiently.
To be considered as a replacement for conventional motive power, to drive vehicles or machinery, however, we need to ask the question: how much CO2 is emitted when we produce the hydrogen. We know that the energy the fuel cell creates is the energy when H and O combine to form water. The energy to have the H, in the first place, is the energy it takes to separate the H and O, from water. As it is evident that the energy economics can never be in favour of the hydrogen fuel cell, the advantage has to lie in using new sources of energy or energy that we would otherwise lose.
The cleanest known way to produce hydrogen is by electrolysis, or by splitting the water molecule using electricity. This could make sense if we created hydrogen at a place where we have electricity, and carry hydrogen to a place where there is no other way. Yes, it could make sense, but if we consider that the bulk of electricity comes from coal fired generation, using hydrogen to create electricity is finally a source of CO2 in the atmosphere.
An alternative would be to use electricity from solar plants. Indeed, this is available, but not in quantity, and if we used it to generate hydrogen, it is at the cost of another application, which must now use coal-based electricity. Another green source could be from bio-energy, or burning agri-waste, which qualifies because the CO2 it gives off is the CO2 it captured when it grew.
To generate hydrogen in quantity, however, we would need to use methods like cracking methane in natural gas or from coal, by splitting water, in the method to create coal gas. These are established and safe methods, already in use in many parts of the world. But these methods emit CO2, and traditionally, this was released into the atmosphere. Now, to create green hydrogen, the CO2 would need to be captured and sequestered. If carbon capture is used with bio-energy, it would be doubly effective, with net reduction of atmospheric CO2. Brown power, based on fossil fuels, would clearly dominate for decades, but hydrogen, and the hydrogen fuel cell, would enable optimising the mix of methods to keep the atmospheric CO2 down
While finding means of generating green hydrogen is the key, the use of hydrogen as a means of heating, fuel for transport and for generating electricity has garnered great interest, world-wide. In the field of electrically powered transport, the fuel cell could replace the storage battery, an expensive component with its own carbon footprint. In India, heavy duty road transport is set to treble by 2040. The technology to power trucks by hydrogen fuel cells is now available. The rise in transport needs is a challenge, but also an opportunity, says a note from TERI (Tata Energy Research Institute).
------------------------------------------------------------------------------------------ Do respond to : response@simplescience.in-------------------------------------------