Hydrogen in place of carbon, is environment friendly., says S.Ananthanarayanan.
Sunlight is finally the only source of all the energy that the earth can use. There is heat stored deep below the surface of the earth, which did not come from the sun, but we have no way to tap this resource. What we need, in these times of energy and environmental crisis, is a way to put sunlight directly to use.
The solar cell and wind energy, are answers, of course. But these are intermittent, and it is not easy to store electricity. Nature’s method is to store solar energy as carbon. Could technology do it better? Soonil Lee, Li Ji, Alex C. De Palma, and Edward T. Yu, from the University of Texas at Austin and Fudan University, Shanghai, write in the journal, Nature Communications, of improvements in devices to use sunlight to extract hydrogen fuel from water. Hydrogen can be used directly as fuel, and it does not produce CO2 on combustion.
Nature’s method of storing sunlight in carbon is by photosynthesis. Green plants, by a miracle of evolution, use sunlight to separate the components of CO2, as carbon compounds and pure oxygen. The carbon remains in wood and plant material, or buried underground as coal and mineral oil. Plants and vegetation humans and animals need as food, as we are carbon-based, but we cannot afford to continue using fossil fuels.
Bio-fuels are considered ‘carbon neutral, because they are made from ‘recent’ plant matter. The carbon released when these fuels are burnt is hence the same as was ‘recently’ absorbed – unlike the case of fossil fuels - which were formed centuries ago. In the same way, methods have been sought to replicate photosynthesis in human-made fuel cells, creating fuel materials from CO2, so that the fuels could also be considered to be carbon neutral. While these are attractive options, cultivation for bio-fuels would need large tracts of land, and artificial photosynthesis is still at the stage of a concept
The serious new contender is hydrogen gas. Although mineral oil fuels, like petrol, contain both carbon and hydrogen, the heat produced by burning a kg of hydrogen is three times the heat from a kg of petrol. Once compressed for use in the fuel tank of a hydrogen-powered vehicle, hydrogen would hence be more efficient, apart from giving off only water vapour as the product of combustion. The only question is, where is the hydrogen to come from? Coal and petroleum are there underground, but hydrogen has to be produced!
The usual methods of producing hydrogen are through electrolysis or when steam is passed over burning coal. The first method needs electricity to work and the second method is itself a source of CO2 emission. It is true that there is electricity from solar cells, but there are other uses for electricity. Should we use it for electrolysis, which is somewhat wasteful, for producing hydrogen? A method of generating hydrogen directly with the help of sunlight is hence what looks like a good solution
The means that we have to put photons of light to work, for the solar cell, or to create hydrogen, is with the help of the silicon crystal. Metals, like copper, gold and silver, are good conductors of electricity because of their atomic structure, that the atoms have fewer electrons in their outermost shell. With most of the electrons in the inner layers almost blocking the attraction of the nucleus, the outer electrons are loosely bound. In the form of crystals, some of these outer electrons float free, and help conduct electricity.
Silicon is an element with a half-way atomic structure, the outer shell has four electrons, which is half-way to the ‘full’ state of eight electrons. The outer shell electrons are somewhat loosely bound, and silicon is called a semiconductor. What this implies is that there are no free electrons, like in metals, but a photon of light can nudge an electron into the free state. This is the property that makes silicon the material used in solar cells, where the electrons released from silicon atoms are harnessed – to generate electricity from light.
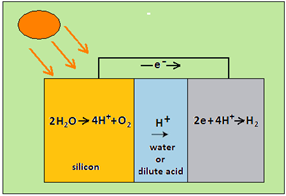
In the hydrogen producing device, called a photoelectrochemical cell, or a PEC cell, a pair of silicon plates is immersed in water, and water acts as a conductor. In the same way as in the solar cell, the silicon exposed to light gives off an electron, and becomes positively charged. While the electron travels to the other plate, the positive charge attracts negative oxygen ions and repels positive hydrogen ions. The hydrogen ions drift to the other plate, where they meet electrons and are released as hydrogen gas. The device is hence a neat way of generating hydrogen, except for the trouble that brews at the first end, where the oxygen has to be dealt with.
Corrosion
At this end, the release of oxygen causes severe corrosion and degradation of the silicon crystal. We have mentioned earlier that metals are elements whose atoms have fewer outer shell electrons. As the ‘complete shell’ is the stable configuration, metals form compounds where the few outer shell electrons are given up. And they are given up to non-metals, like oxygen or sulphur, the atoms of whose outer shells have more electrons and can gain a few more, to become ‘complete’. The silicon plate where oxygen is released hence reacts with oxygen and is rapidly corroded.
The strategy has to save the silicon plate been to protect the surface, and the method used is with the metal-insulator-semiconductor (or MIS) structure. A thick insulating layer would give good protection, but would also impede the splitting of the water molecules and release of hydrogen. “Typically, there is a tradeoff between efficiency and stability when optimizing insulator thickness,” says the paper in Nature Communications, as the context of the workaround that the authors propose.
One material that is used as the insulator is a layer of silicon dioxide, which oxygen cannot corrode, and would protect the silicon surface. A layer that would allow the PEC cell to work, however, needs to be just nanometers thin, which is not enough, as a protection. What the authors of the paper have devised, is to provide conduction pathways through a thick layer of SiO2, to allow electrical conduction, but still protect the surface.
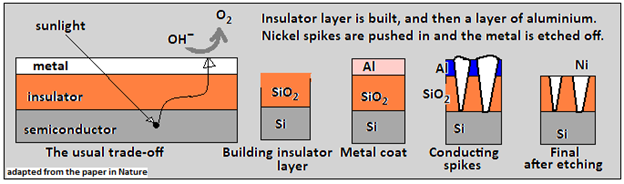
The paper says one solution followed was to use a thick insulator layer, but create areas where the insulation broke down, to allow conduction. This process did work, but was complex, the paper says. What the team has done instead, is to build a thick insulating layer over the silicon slab and then overlay with a thin film of aluminum. Annealing the structure, at 550°C, for 24 hours, leads to spikes of aluminum going through the insulator, to create conducting channels. The aluminum is now etched away, and the spikes filled with nickel, which is conducting, as well as a catalyst that promotes the splitting of H2O.
The procedure is low-cost and can be employed on a large scale. And the results are generation of hydrogen a low intensity light exposure, high capacity and excellent stability, the paper says. With hydrogen driven cars, buses and truck, even trains, being developed, an efficient and sustainable source of hydrogen would hasten their wider acceptance.
------------------------------------------------------------------------------------------ Do respond to : response@simplescience.in-------------------------------------------