S.Ananthanarayanan speaks of tools for tailoring DNA.
In the year 2001, Stephen Hawking said we had entered a century of biotechnology. The last two years, if the pandemic should be thanked for anything, have raised public understanding of the internals of genetics, the virus, the DNA. And the field of genetic engineering, with methods of manipulating the DNA itself, has become the central technology of our times.
This was not the case at the start, however. The start was in 1953, with the discovery of the structure of DNA, by Francis Crick, James Watson and Maurice Wilkins, based on the seminal work of Rosalind Franklin. And this explained how the complementary pair of strands, that DNA consists of, split apart at the time cell division, so that the two halves could recreate the whole in the daughter cells. And just over a decade later, Robert Holley, Marshall Nirenberg and Har Gobind Khorana uncovered the mechanism by which the millions-of-units-long DNA encoded and passed on the templates for the 20 amino acids that all proteins consist of.
This was a start, but the nature of DNA was still far from understood. It was known that the DNA molecule was a series of units that came from only four chemical groups, called bases. And it was known that the four bases formed base pairs that appeared together. The pattern, or the order of the appearance, over the length of the DNA molecule, however, was out of reach.
The task of mapping out the entire structure of the human DNA, or the genome, was undertaken in 1990, with 2,800 researchers working, worldwide, and was completed in 2003. The structure worked out is of 3 billion base pairs, in about 20,500 groups, called genes, which code for proteins. This was the progress made in the first 50 years since 1953. But the progress since 2003 has been rapid and we are now able to identify the structure, down to base pairs, of the genes responsible for diseases, and then, in cases, repair errors that have appeared. The last two years have shown us the capability to build, in the laboratory, versions of segments of the DNA, to create vaccines that can train the immune system to recognize the SARS-CoV2 virus.
This capacity is due, to a large extent to a procedure, called CRISPR-Cas9, developed by Jennifer Doudna and Emmanuelle Carpentier in 2016. While genetic engineering is set to modify natural processes for improved health, environment control and industrial processes, CRISPR-Cas9 was developed by mimicking a natural process, where bacteria make use of genetic engineering in defense against viruses.
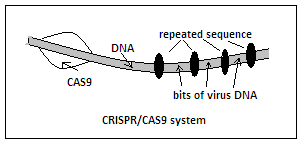
The discovery started with the observation that the DNA of many bacteria contains a series of sequences that are repeated, interspersed with sequences that are derived from viruses. And it was noticed that the repeats usually occurred in the vicinity of another set of genes, of a kind that seemed to be involved in the process of DNA repair. It was then thought that the repeats were perhaps some kind of template acquired from encounters with viruses and later used for resisting the same viruses. The repeats were named, CRISPR, a short form for Clusters of Regularly Interspaced Short Palindromic Repeats, and the associated genes were called CAS genes, for CRISPR Associated genes.
While the DNA are the construction formula of all the proteins that the organism would need, the whole DNA is never active at any one time. Instead, it is portions of the DNA, that represent the code for just a set of proteins, that are extracted and utilised for creating different proteins that define the nature of a cell. The medium to transmit the partial code to the cells’ engines that build the proteins is the RNA, a variation of the DNA molecule structure. Now, when a virus attacks a bacterium, the identifying sequences of the virus get copied on to scraps of RNA which form during the encounter with the virus and these get stored in the CRISPR sequences. When the virus attacks again, it is not a passive action of taking and storing copies of parts of the virus DNA that happens, but the existing patterns of the virus DNA are copied quickly on to RNA, which then combine with proteins, or enzymes, that are formed from the CAS genes portion, and an important group have been called CAS9.
CAS9, equipped with the template for a portion of the virus, is able to zero in to the portion of the virus DNA that CRISPR has recorded and CAS9 acts by snipping the DNA at that place. The virus, which consists mainly of its own DNA, is then destroyed and the bacterium stays healthy.
In 2012, scientist Jennifer Doudna, of the University of California at Berkley, and her colleague Emmanuelle Carpentier, and their team, studied the process of this action and they identified CAS9 as the enzyme which could cause a rupture in the two strands of the DNA molecule at the portion marked by the pattern of the RNA, called the guide RNA. And when Doudna and Carpentier got down to how this happened in the bacteria, they wondered if the process could be mimicked with a given RNA to serve as the guide RNA, to create a system that could sever DNA at the place specified by the guide RNA.
The team went on to do this and then they devised a test to see if the target DNA had actually been cloven at the point they wanted. They first generated a pair of guide RNA that matched specific parts of a known DNA molecule and then formed the cleaving enzymes by letting the RNA attach to CAS9. The target DNA was then incubated with the cleaving enzymes, and then, the DNA was examined with the help of a gel in which segments of DNA move with different speeds. The gel was able to separate two parts of the DNA and the sizes of the portions showed that the original DNA had been cut just where the cleavage was programmed by the guide RNA.
Studies had shown for some time that cells had ways of repairing DNA damage. In such cases, when a DNA was separated, the two portions rejoined, either just as they had broken or with a small section added, or there could be the addition of a whole part of DNA which matched the two broken ends. Where the DNA rejoined in the ordinary way, there was often damage to the gene involved, which had its value in studying the properties or function of that gene. Where a portion was added, the genome now had additional genes, with their value in research. The CRISPR and CAS9 system which allow DNA to be divided accurately, and then modified, has made possible many forms of DNA manipulation. The technique is said to be “igniting a revolution across the life sciences” and is quickly becoming a standard tool in many labs.
More uses of CRISPR technology
The use of CRISPR has gone beyond the traditional gene knockouts and knock-ins.
New technologies include CRISPRi, or CRISPR interference – where the DNA is not cleaved, but the functioning of a gene is suppressed. Doing this with genes in succession enables identifying gene function.
Another is CRISPRa, or CRISPR activation – where genes are made more active. An application is where a gene is switched on by blue light and repressed when the light is turned off.
Yet another is the anti-CRISPR protein, which enables a gene to evade CRISPER action.
CRISPR screens allow inhibition of a group of genes, gene tagging helps attach a marker to specific genes, and prime and base editing allow changes to be made within individual DNA components.------------------------------------------------------------------------------------------ Do respond to : response@simplescience.in-------------------------------------------